June 8 2021 100539 am. The Drugs Controller General of India DCGI had cleared the formulation on May 1 for emergency use as an adjunct therapy in moderate to severe Covid-19 patients.

Covid 19 Bulletin 07 June 2021 E
COVID-19 patients may be required to take this drug for 5-7 days to completely stop viral growth.

Medicine for covid 19 by drdo. As India battles the second wave of Covid infections an anti-Covid drug found to help speed up recovery and reduce. The drug 2-deoxy-D-glucose has been used in clinical trials to stop growth of cancer cells while it helped decreasing oxygen dependence in COVID patients. Directions for the usage of this drug for COVID-19 patients as per DCGI are below.
DRDOs Covid-19 drug to be available for Rs 990 commercial launch in mid-June The price of DRDOs 2DG anti-COVID 19 drug has. List of these products has been given below. Once the growth is inhibited there will be no sudden rise in the.
DRDOs 2DG medicine to treat Covid-19. Dr Sudhir Chandna Institute of Nuclear Medicine and Allied Sciences INMAS scientist has said the recently approved anti-COVID-19 drug 2-deoxy-D-glucose 2-DG is completely safe and will help patients recover faster. 15052021 In a good news for coronavirus patients DRDO-developed 2DG medicine for treating COVID-19 patients will be made available from next week.
15062021 The drug developed by DRDO in collaboration with Hyderabad-based Dr Reddys Laboratories was approved by the Drugs Controller General of India DGCI last month for emergency use as an adjunct. Availability dosage price 2DG will work against variants too as it stops virus growth. Development Organisation DRDO said on May 14.
Right now the drug has been recommended for use in moderate to severe COVID-19 cases only. 18052021 DRDOs New Covid Drug 2-DG should also work against various strains of Covid. 17052021 The 2DG medicine is to be taken orally mixed in water 2 times a day.
DRL has been a long. 21062021 DRDO official told Financial Express Online that DRL was the development partner with DRDO in the study on COVID-19 patients. Explained Desk New Delhi Updated.
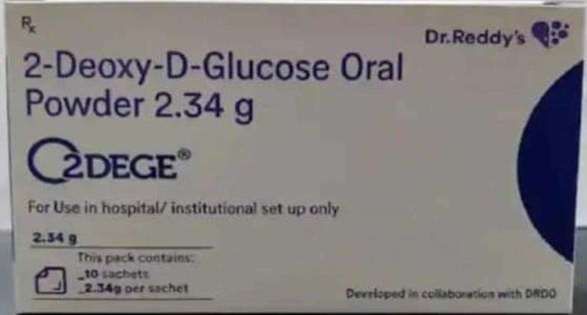
10052021 DRDOs new anti-Covid oral drug 2-deoxy-D-glucose 2-DG was recently granted emergency use approval by the Drug Controller General of India DCGI as an adjunct therapy in moderate cases of. An anti-COVID-19 therapeutic application of the drug 2-deoxy-D-glucose 2-DG has been developed by Institute of Nuclear Medicine and Allied Sciences INMAS a lab of Defence Research and Development Organisation DRDO in collaboration with Dr Reddys Laboratories DRL Hyderabad. Diitm athqr dotdrdo dotin.
08052021 DRDO-developed anti-Covid 19 drug gets DCGI approval for emergency use An anti-COVID oral drug developed by the DRDO has been approved by the Drugs Controller General of India DCGI for emergency use as an adjunct therapy in moderate to severe coronavirus patients the defence ministry said on Saturday. 011 23007443 011 23007445 011 23013209. ShachiboltaayBy - Shachi Sutaria.
11052021 New anti-Covid-19 drug 2-DG in market in three weeks. DRDO has developed many products for combating COVID-19. Dr Shiv kumar Director ER.
08062021 DRDO 2-DG Medicine. 15052021 The first batch of 2DG medicine for treating COVID-19 patients would be launched early next week Defense Research. Interested agencies persons before approaching industries directly may kindly contact.
DRDOs 2-DG Medicine As Additional Therapy For COVID-19. 08052021 Anti-COVID-19 oral drug developed by DRDO Dr Reddys approved for emergency use Clinical trials of the drug 2-deoxy-D-glucose 2-DG. The Defence Research and Development Organisation DRDO on Tuesday issued directions for the use of 2DG 2-Deoxy-D-glucose drug for Covid-19 patients under care as per Drugs Controller General of India DCGI approvalThe drug can be given on a prescription by a doctor.
2-DG has been developed by INMAS a lab of Defence Research and Development Organisation DRDO in collaboration with Dr Reddys. Drugs Controller General of India DCGI had approved this anti-COVID oral drug for emergency use last week on May 8. Satheesh Reddy outlines his organisations role in the fight against Covid-19 in this interview with Executive Editor Sandeep Unnithan.
Speaking to ANI DRDO officials said that as many as 10000 doses of the drug would be administrated across India. DRDO chief From drugs to setting up medical oxygen plants DRDO chairman Dr G.

New Covid Drug 2 Dg By Drdo Launched How 2 Deoxy D Glucose Medicine Fights Covid 19 Youtube
India Siapkan Obat Oral Anti Covid 19 Melawan Semua Varian Virus Corona Begini Cara Kerjanya Semua Halaman Grid Health

Dcgi Nods Anti Covid Drug Developed By Drdo For Emergency Use

Drdo S 2 Dg Drug For Covid Patients Available From Next Week The Federal
Phase 3 Clinical Trials For 2 Dg May Continue Till August Suggests Registry Data

Coronavirus Crisis Drdo Successfully Tests Multi Patient Ventilator Bhel To Make 5000 By April End Youtube

Drdo S Anti Covid 19 Drug 2 Dg Full Detailed Information In Hindi Drdo Youtube
Drdo Scientist Anant Narayan Bhatt Explains How 2dg Drug Helps Body To Fight With Covid 19
First Batch Of Drdo S Anti Covid Drug 2 Dg To Be Released Tomorrow Latest News India Hindustan Times

0 comments:
Post a Comment