For anyone under age 18. 30032020 In October 2020 the FDA approved the antiviral drug remdesivir to treat COVID-19.

Can Chloroquine And Hydroxychloroquine Treat Coronavirus Covid 19 Goodrx
Health authorities will be implementing more testing for passengers.

Covid medicine for 1 year old. Two the Moderna and Johnson. 13012021 On May 10 2021 the FDA expanded its emergency use authorization EUA for the PfizerBioNTech COVID-19 vaccine to include adolescents 12 to 15 years old. 18062021 Currently the Pfizer vaccine is only approved for children 12 and older So far 5 to 11-year-olds have received their first shot.
03062021 A two-year-old boy who was hospitalised after testing positive for coronavirus is now in an Adelaide medi-hotel SA Health says. 09112020 Kids and families can reduce coronavirus risk together. 26102020 New research suggests that an antimalarial an anti-psychotic drug and a drug that helps control blood pressure may be repurposed as COVID-19 treatments.
Clinical trials suggest that in these patients remdesivir may modestly speed up recovery time. Your child will need a second shot of the Pfizer-BioNTech COVID-19 Vaccine 3 weeks after their first shot. Next up are 2 to 4-year-olds followed by infants 6 months up to toddlers two years old.
05062020 While COVID-19 tends to be less severe in children this protection does not extend to babies who may be as vulnerable to severe illness as older adults. Here is what you need to know about the COVID-19 vaccine for children including when pharmaceutical manufacturers expect the vaccine to be available for those younger than 12. 08052020 Ibuprofen which is also known by the brand names Advil and Motrin is a non-steroidal anti-inflammatory drug NSAID.
For now this is the only vaccine authorized in the US. These types of medications can help lower your fever and minimize muscle aches from COVID-19 while also reducing some inflammation in your body. The drug may be used to treat adults and children ages 12 and older and weighing at least 88 pounds who have been hospitalized for COVID-19.
11022020 Babies under 1 year old might be more likely to have severe illness from COVID-19. Other children regardless of age with the following underlying medical conditions might also be at increased risk of severe illness compared to other children. 15052021 Get a COVID-19 vaccine for your child as soon as you can.
23032021 Use the COVID-19 vaccine eligibility checker to find out when and where you can receive a COVID-19 vaccine. If you are aged 40 years or more you are eligible for vaccination. If you are aged 16 to 39 years you may be eligible for vaccination.
04062021 Initially the vaccines were only available to people age 16 and older. History which includes studies in adolescents. 12052021 There are three COVID-19 vaccines currently authorized for emergency use in the US.
A Chinese analysis that included more than. COVID-19 vaccines have been used under the most intensive safety monitoring in US. Young Black and Hispanic men and women are more at risk than their white counterparts due to longstanding racial.
COVID-19 vaccines are safe and effective. People under 16 years of age are not able to get vaccinated at this time. But on May 10 2021 the Food and Drug Administration issued an emergency use authorization for the Pfizer-BioNTech COVID-19.
The process was pretty simple and he sat still for most of it. Ibuprofen doesnt treat the virus itself but it can make you feel a lot better. Though COVID-19 seems to have less serious health consequences for children than for adults it is important to avoid infection among children ensure they get their flu shots and other vaccinations be on the lookout for serious disease in kids and help prevent the virus from spreading.
Data from one study shows that of more than 3000 adults ages 18 to 34 who contracted COVID-19 and became sick enough to require hospital care 21 ended up in intensive care 10 were placed on a breathing machine and 27 died. 30042021 The two-dose vaccine is already authorized for use in people 16 and older. 01042020 Nearly a week after COVID-19 diagnosis 1-year-old is recovering happy The low-grade fever and crankiness seemed like something that could happen to any toddler.
09042021 I took my toddler to get a rapid nasal-swab test for COVID-19. 04062021 In general childrens risk of becoming ill with Covid-19 is extremely low and they very rarely need hospital treatment which is why the focus has. Earlier this month Pfizer and BioNTech asked the FDA to allow their Covid.
Asthma or chronic lung disease. Everyone that had passed away from COVID early on didnt get a chance to even get the vaccine. Food and Drug Administration has issued emergency use authorization of the Pfizer-BioNTech COVID-19 vaccine for children 12 to 15 years old.
When her fever spiked and she. JohnonJanssen Pharmaceuticals vaccines are approved for adults 18 and older while the Pfizer-BioNTech vaccine is approved for anyone 12 and up. Previously the Pfizer vaccine was authorized for use in children 16 years and older.

Aiims Issues New Guidelines For Treatment Of Covid 19 Cases Coronavirus Outbreak News

News Or News2 Score When Assessing Possible Covid 19 Patients In Primary Care The Centre For Evidence Based Medicine
Icu Outcomes And Survival In Patients With Severe Covid 19 In The Largest Health Care System In Central Florida

Covid 19 Q A Kids Health University Of Maryland Children S Hospital

N Acetylcysteine A Rapid Review Of The Evidence For Effectiveness In Treating Covid 19 The Centre For Evidence Based Medicine

Covid Vaccine Max At Rs 250 Dose In Private Hospitals Free In Govt Ones Check Eligibility How To Get It Coronavirus Outbreak News

Baricitinib Therapy In Covid 19 Pneumonia An Unmet Need Fulfilled Nejm
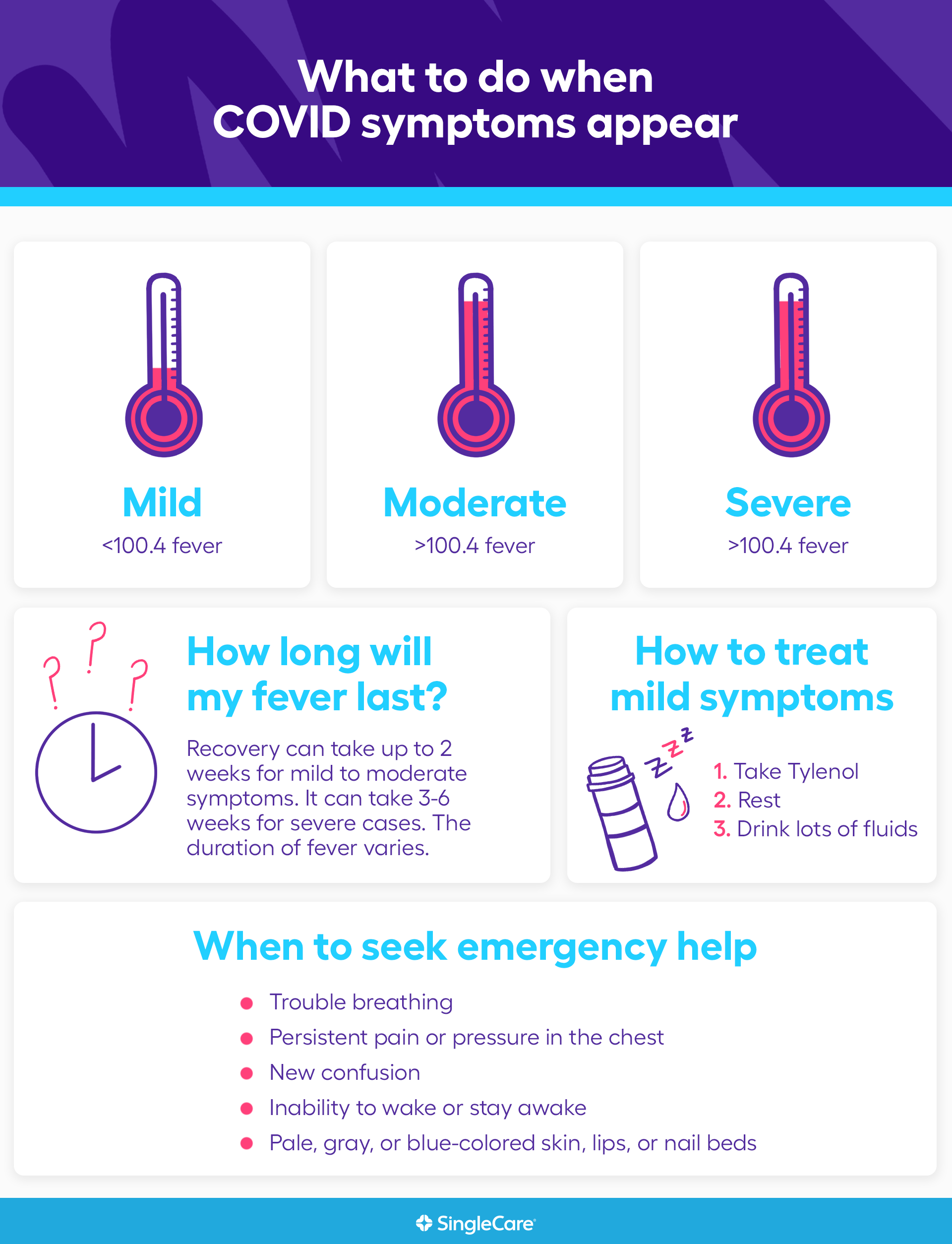
Coronavirus Symptoms Mild Moderate Severe


0 comments:
Post a Comment